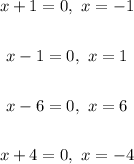GIVEN:
We are given the following polynomial;
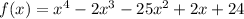
Required;
We are required to sketch the graph of the function. Also, to use the synthetic division and the remainder theorem to find the zeros.
Step-by-step solution;
We shall begin by sketching a graph of the polynomial function.
From the graph of this polynomial, we can see that there are four points where the graph crosses the x-axis. These are the zeros of the function. One of the zeros is at the point;
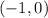
That is, where x = -1, and y = 0.
We shall take this factor and divide the polynomial by this factor.
The step by step procedure is shown below;
Now we have the coefficients of the quotient as follows;
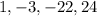
That means the quotient is;
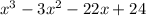
We can also divide this by (x - 1) and we'll have;
We now have the coefficients of the quotient after dividing a second time and these are;
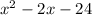
The remaining two factors are the factors of the quadratic expression we just arrived at.
We can factorize this and we'll have;
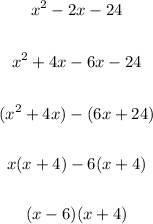
The zeros of this polynomial therefore are;
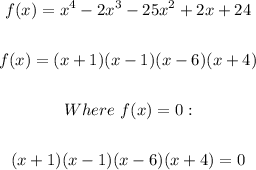
Therefore;
ANSWER: