Given:
A lab assistant needs to create a 1000 ML mixture that is 5% hydroelectric acid.
The assistant has solutions of 3.5% and 6% in supply at the lab.
let the number of milliliters from the solution of 3.5% = x
And the number of milliliters from the solution of 6% = y
so, we can write the following equations:
The first equation, the sum of the two solutions = 1000 ml
So, x + y = 1000
The second equation, the mixture has a concentration of 5%
so, 3.5x + 6y = 5 * 1000
So, the system of equations will be as follows:
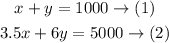
Now, we will find the solution to the system using the substitution method:
From equation (1)
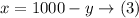
substitute with (x) from equation (3) into equation (2):
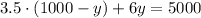
Solve the equation to find (y):
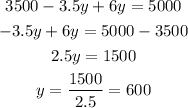
substitute with (y) into equation (3) to find x:

So, the answer will be:
Enter the equations below separated by a comma
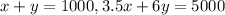
How many milliliters of the 3.5% solution should be used?
400 milliliters
How many milliliters of 6% solution should be used?
600 milliliters