To determine the percentage of oxygen in the molecule we must first know its structure and how many oxygen atoms are contained in it. The structure of aluminum phosphate is AlPO4.
It means that there are 4 oxygen atoms, 1 Aluminum atom, and 1 phosphate atom.
Now we will determine the weight of the molecule AlPO4 by adding the atomic weights of the elements as follows:
Element Atomic mass # of atoms Mass
Al 26.9815 1 26.9815
P 30.9738 1 30.9738
O 15.999 4 63.996
Total mass of AlPO4 = 26.9815+30.9738+63.996 = 121.9513 g/mol
Now we will determine the mass percentage with the following equation:
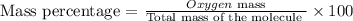
We replace the known terms:
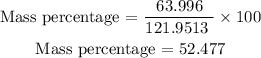
So, the percentage of oxygen in aluminum phosphate is 52.477%