Answer:
10.7 grams
Explanations:
The formula for calculating the molarity of a solution is given as:

Given the following parameters
molarity of NaOH = 1.11M
volume of solution = 241.0mL = 0.241L
Find the moles of NaOH
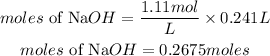
Determine the mass of solute (NaOH)
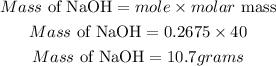
Hence the grams of solute that would be used to prepare the solution is 10.7grams