Answer
a. 74.2
Step-by-step explanation
Given that:
The initial temperature, T₁ = 63.2 °C + 273 = 336.2 K
Initial pressure, P₁ = 57.3 kPa
The final volume, V₂ = 15.56 mL
Final temperature, T₂ = 138.7°C + 273 = 411.7 K
Final pressure, P₂ = 334.6 kPa
What to find:
The initial volume, V₁.
Step-by-step solution:
The initial volume, V₁ can be calculated using the combined gas law equation.
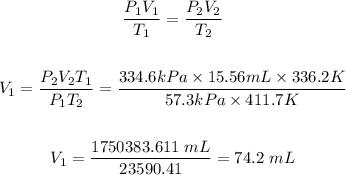
Hence, its initial volume at 63.2 °C and 57.3 kPa is 74.2 mL