Answer:
0.167L
Step-by-step explanation:
In order to know how much L of the 3M NaOH solution would you need, we will simply set up an equal proportion solution expressed as;
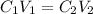
C1 and C2 are the concentration of the solutions
V1 and V2 are the volumes of the solutions
Given the following parameters;
C1 = 1M
V1 = 500mL
C2 = 3M
V2 = ?
Substitute the given parameters into the formula above to get the required litre of solution needed.
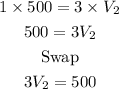
Divide both sides by 3:
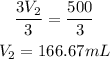
Converting to litres
Since 1mL = 0.001L
166.67mL = x
Cross multiply
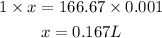
Hence the amount of L of the 3M NaOH solution would you need is 0.167L