Explanations:
Given the balanced chemical reaction expressed as:
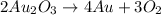
A Redox reaction is a reaction that involves the transfer of electrons and changes in the oxidation state between the elements.
The given chemical equation is therefore an oxidation-reduction reaction since it involves a change in the oxidation state of the elements.
Determine the moles of Au₂O₃
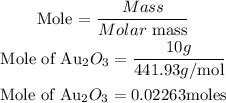
According to stochiometry, you can see that 2 moles of Gold(III)oxide produce 4 moles of Gold. Hence the moles of Gold produced will be:
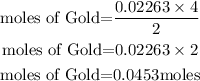
Determine the mass of Gold.
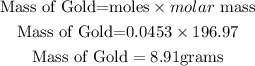
Next is determining the mole of Oxygen
According to stochiometry, you can see that 2 moles of Gold(III)oxide produce 3 moles of Oxygen. Hence the moles of Oxygen produced will be:
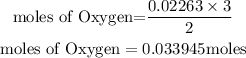
Determine the mass of oxygen produced
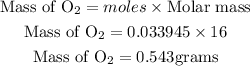
Hence the mass of oxygen produced is 0.543 grams