Given:
A cylinder truck all paint cans to be inches across the top diameter in about 10 inches high.
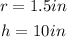
Required:
To find the volume of the cylinder.
Step-by-step explanation:
The volume of the cylinder is,
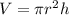
Therefore,
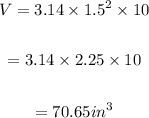
Final Answer:
70.65 cubic inches of paint it hold.