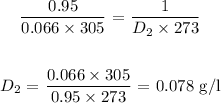Answer:
Step-by-step explanation:
Here, we want to calculate the density of the gas at STP
We use a modification of the general gas law as follows
Mathematically:
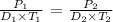
where:
P1 is the initial pressure which is 0.95 atm
D1 is the initial density which is 0.066 g/l
T1 is the initial temperature in Kelvin (we add the temperature in Celsius with 273 K : 32 + 273 = 305 K)
P2 is the pressure at STP which is 1 atm
D2 is the density that we want to calculate
T2 is the temperature at STP which is 273 K
Substituting the values, we have it that: