To find the number of molecules we must apply for Avogadro's number. This number tells us that in one mole of any substance there are 6.022x10^23 molecules. So the first thing we will do is find the moles contained in 200 grams of C6H12O6 (Glucose).
The molar mass of glucose is 180.06g/mol. So, the moles of glucose will be:
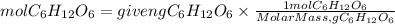
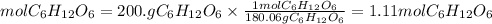
Now, we apply for Avogadro's number. So, the molecules of glucose will be:
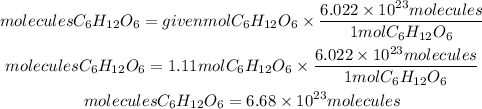
Answer: In 200 grams of C6H12O6 there are 6.68x10^23 molecules