We know that the amount of matter is given by:
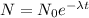
where λ is the decay constant. The decay constant is related to the half-life of the element by the equation:
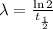
Then we can express our first equation as:
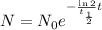
Plugging the initial amount, 50 g, the half-life of 5715 years and the time we want to know we have that:
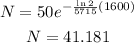
Therefore, after 1600 years there are 41.181 g