Answer
101.3 mL
Step-by-step explanation
Given:
The initial temperature, T₁ = 148.5 °C = (148.5°C + 273) = 421.5 K
The initial volume, V₁ = 241.8 mL
Final temperature, T₂ = -96.4 °C = (-96.4°C + 273) = 176.6 K
What to find:
The final volume of the gas.
Step-by-step solution:
The final volume, V₂ of the gas can be calculated using Charle's law formula.
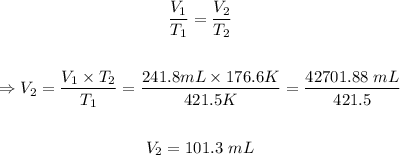
The final volume of the gas is 101.3 mL