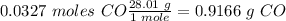Answer
0.9166 g CO
Procedure
Considering the conditions and parameters given ideal gas law will be assumed.

The gas constant is 8.3144 L⋅kPa⋅ K⁻¹⋅mol⁻¹
Converting the current data to the required units
710 mL = 0.710 L
37 °C = 310.15 °K
Solving for moles and substituting the variables with the available data
![n=(PV)/(RT)=\frac{119\text{ }kPa(0.710)L}{8.3144\text{ L. kPa\cdot}\degree\text{K^^^^207b^^b9\cdot mol^^^^207b^^b9 \lparen310.15\rparen }\degree K}=0.0327\text{ }moles\text{ CO}]()
Transforming from moles to grams using the molecular weight