nswer
C. Cr₂O₃ (s)
Step-by-step explanation
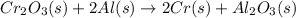
An oxidizing agent is a substance that causes oxidation by accepting electrons; therefore, its oxidation state decreases.
A reducing agent is a substance that causes reduction by losing electrons; therefore its oxidation state increases.
The simplest way to answer this is that the oxidizing agent is the substance that is reduced, while the reducing agent is the substance that is oxidized.
Therefore, CrA