Step 1 -
A decomposition reaction is a reaction in which a compound is decomposed, i.e., we usually heat a substance thus forming new substances, such as gaseous substances.
Generally speaking, a decomposition reaction will involve only one reactant: the substance to be decomposed.
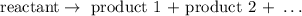
Step 2 - Examples of decomposition reactions
Let's take a closer look at some examples:
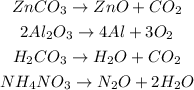
We can already state some conclusions:
a) The products are not always the same. They depend on the reactant. Therefore, they are not always predictable.
b) The reactant can be an ionic compound (such as ZnCO3) but also a covalent compound (such as H2CO3).
c) The reactant does not decompose into its constituent elements.
Step 3 - Finding the wrong answer
Therefore, the only wrong answer is item b), the products are the constituent elements.