Step-by-step explanation
o solve this , we need to use the formula:
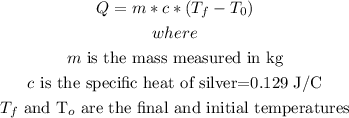
so
Step 1
) Let
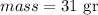
so, we need to convert from grams to kilograms ,to do that, divide the amount in grams by 1000
so
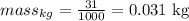
and
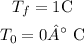
b) now, replace in the formula
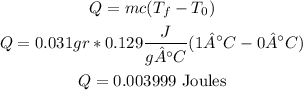
so, the answer is 0.003999 Joules
hope this helps you