Answer:
C. Atom Y
Step-by-step explanation:
when calculating charge, the "Number of Neutrons" doesn't matter because neutrons have no charge. (so you can ignore the neutrons section for this question)
also, protons have a positive charge, and electrons have a negative charge
to solve this problem, add the charges of the protons and neutrons together to see if the number ends up positive (because question is looking for positive charge)
for Atom W:
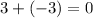 , so no, this isn't the answer. 0 is neutral charge
, so no, this isn't the answer. 0 is neutral charge
for Atom X:
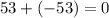 , this isn't the answer either. 0 is neutral charge
, this isn't the answer either. 0 is neutral charge
for Atom Z:
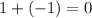 , nope, 0 is neutral charge
, nope, 0 is neutral charge
for Atom Y:
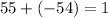 , yay this is it, 1 is positive which indicates positive charge.
, yay this is it, 1 is positive which indicates positive charge.
therefore, C. Atom Y is the answer