Answer:
 .
.
Step-by-step explanation:
1. Find the molar mass of copper.
Using the periodic table, we are able to obtain the molar mass of copper:
63.546 g/mole.
There are, approximately, 6.022*10^23 atoms of any element in one mole of that element. This means that there are 6.022*10^23 atoms in 63.546 grams of copper. Hence, we may construct a rule of 3 to answer the question.
2. Construct a rule of 3.
g -------------- 6.022*10^23 atoms
150.0g ---------------- x
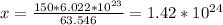
Therefore, the amount of atoms that there are in 150g of copper is
 .
.