Answer:
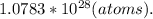
Step-by-step explanation:
Since I don't have access to "Appendix A", I'll solve the problem using data from the periodic table.
1. Determine the molar mass of iron.
According to the periodic table, the molar mass of iron is:
55.845g/mole.
2. Convert 1 tonne to grams.
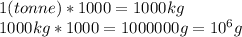
3. Apply rule of 3.
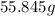 -----------
-----------
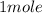
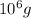 -----------
-----------

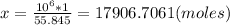
4. Determine the amount of atoms.
Considering that there are, approximately,
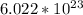 atoms in a mole of any element, apply another rule of 3.
atoms in a mole of any element, apply another rule of 3.
1 mole ---------------------
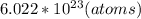
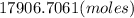 --------------------- x
--------------------- x
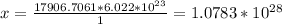 .
.