Answer:
= 1.553 x 10²³ ions
Step-by-step explanation:
One mole of a compound or a substance consists of 6.02 x 10²³ ions. 6.02 x 10²³ is also referred to as Avogadro's number/constant.
From the above question, we have been asked to determine the number of ions in 16g of H₂CO3.
The relative formula mass (RFM) of H₂CO3 is 62.026 u:
Derived as;
H₂CO3 = (2 x 1.008) + (12.01 x 1) + (3 x 16.00) u
= 62.026 u.
Number of moles = Mass/RFM
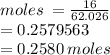
1 mole = 6.02 x 10²³ ions
0.2580 moles = ?
=
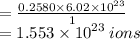
Hence the number of ions in 16g H2CO3 = 1.553 x 10²³