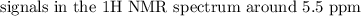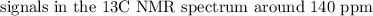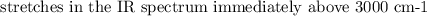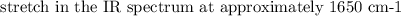Answer:
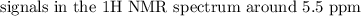
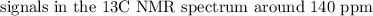
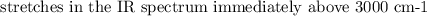
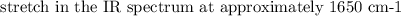
Step-by-step explanation:
The spectroscopy of distributed alkenes is adequately not the same as those of alkanes in many occurrences to make it conceivable to perceive when a double bond is available. For instance, in the infrared range of 1-butene, the assimilation band close to 1650cm−1 is normal for the extending vibration of the double bond.
As a rule, the power and position of this band rely upon the design of the alkene; it changes with the level of at the double bond, with the presence of a second unsaturated gathering in formation with the first, and with the evenness of the replacement of the double bond.
SO, from the information given: the correct information is: