Answer:
Option B.
Step-by-step explanation:
- 2Pb(NO₃)₂ → 2PbO + 4NO₂ + O₂
First we convert 9.90 g of Pb(NO₃)₂ into moles, using its molar mass:
- 9.90 g ÷ 331.2 g/mol = 0.0299 mol Pb(NO₃)₂
Then we convert moles of Pb(NO₃)₂ into moles of PbO, using the stoichiometric coefficients:
- 0.0299 mol Pb(NO₃)₂ *
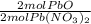 = 0.0299 mol PbO
= 0.0299 mol PbO
Now we convert 0.0299 mol PbO into grams, using its molar mass:
- 0.0299 mol PbO * 223.2 g/mol = 6.67 g PbO
So 6.67 g is the theoretical yield. With that in mind we calculate the percent yield:
- % yield = 5.51 / 6.67 * 100 % = 82.6%
So the correct answer is option B.