Answer:
a) The coordinates of the missing vertex = (7, 8)
b) Area = 18 square units
Perimeter = 18 units
Explanation:
a) We know three of the four vertices:
A: (4, 2) C ______ D(?)
B: (7, 2) | |
C: (4, 8) A |______| B
To find the coordinates of the missing vertex we need to calculate the distance in the x-direction from point A to point B:

Hence, the distance of point D from point C in the x-direction is:
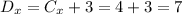
Now, to find the coordinate in "y" we need to calculate the distance in the y-direction between point C and point A:
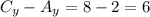
Then, the distance of point D from point B in the y-direction is:
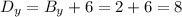
Therefore, the coordinates of the missing vertex (point D) are:
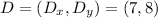
b) The area of the rectangle is:
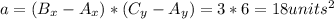
The perimeter is given by:
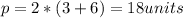
I hope it helps you!