Answer:
![5.6dm^3[/tex[ of gas is produced when 0.1 moles of magnesium nitrate is decomposed.</strong></p><p><strong>Explanation:</strong></p><p>The balanced chemical equation is: </p><p>[tex]2NH_4NO_3(s)\rightarrow 2MgO(s)+4NO_2(g)+O_2(g)](https://img.qammunity.org/2022/formulas/chemistry/high-school/yid8v722cwbozr2v3wx3a6wq98bytmnyjs.png)
According to stoichiometry :
2 moles of
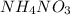 produce = 4 moles of
produce = 4 moles of
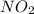 gas and 1 mole of
gas and 1 mole of
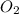 gas
gas
2 moles of
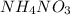 produce = 5 moles of gas
produce = 5 moles of gas
Thus 0.1 mole of
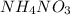 produce =
produce =
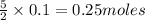 of gas
of gas
Volume of gas produced =
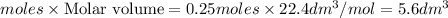
Thus [tex]5.6dm^3[/tex[ of gas is produced when 0.1 moles of magnesium nitrate is decomposed.