Answer:
Kp = 1.41 x 10⁻⁶
Step-by-step explanation:
We have the chemical equation:
2 A(g) + 3 B(g)⇌ C(g)
In which A and B are the reactants and C is the product. We calculate first the change in the number of moles of gas (Δn or dn):
dn= (sum moles products - sum moles reactants)
= (moles C - (moles A + moles B))
= (1 - (2+3))
= 1 - 5
= -4
We have also the following data:
Kc = 63.2
T= 81∘C + 273 = 354 K
R = 0.082 L.atm/K.mol (it is a constant)
Thus, we introduce the data in the mathematical expression for the relation between Kp and Kc:
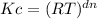 = (0.082 L.atm/K.mol x 354 K)⁻⁴ = 1.41 x 10⁻⁶
= (0.082 L.atm/K.mol x 354 K)⁻⁴ = 1.41 x 10⁻⁶