Answer:
The resulting pressure is 300 kilopascals.
Step-by-step explanation:
Let consider that gas within the closed vessel behaves ideally. By the equation of state for ideal gases, we construct the following relationship for the isothermal relationship:
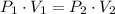 (1)
(1)
Where:
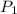 ,
,
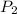 - Initial and final pressure, measured in kilopascals.
- Initial and final pressure, measured in kilopascals.
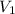 ,
,
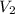 - Initial and final volume, measured in litres.
- Initial and final volume, measured in litres.
If we know that
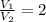 and
and
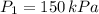 , then the resulting pressure is:
, then the resulting pressure is:
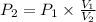
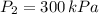
The resulting pressure is 300 kilopascals.