Answer:
a.
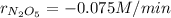
b.
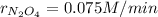
Step-by-step explanation:
Hello.
In this case, according to the balanced chemical reaction, we can write the law of rate proportions:
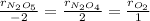
Thus, we proceed as follows:
a. Since the rate of oxygen production is 0.15 M/min, we can make the following setup:
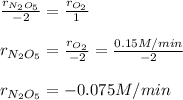
b. Since the rate of oxygen production is 0.15 M/min, we can make the following setup:
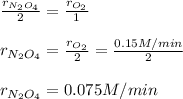
Best regards!