Answer: (i) blue colour fades away
(ii) Single replacement
(iii) copper sulphate + iron
 iron sulphate + copper
iron sulphate + copper
Step-by-step explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
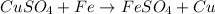
Here iron being more reactive than copper can easily displace copper from its salt solution and form iron sulphate and copper in elemental form.
The blue colour of the copper sulphate solution fades away.
The word equation is :
copper sulphate + iron
 iron sulphate + copper
iron sulphate + copper