Answer:
The electrical force between the electrons and protons in the atom is 6.62 x 10⁻⁸ N.
Step-by-step explanation:
Given;
distance between the electron and proton, r = 5.9 x 10⁻¹¹ m
charge of electron, q₁ = -1.6 x 10⁻¹⁹ C
charge proton, q₂ = +1.6 x 10⁻¹⁹ C
The electric force between the two particles is calculated by Coulomb's law;
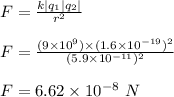
Therefore, the electrical force between the electrons and protons in the atom is 6.62 x 10⁻⁸ N.