Answer:
a. Zinc is the limiting reactant.
b.
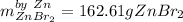
c.
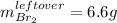
Step-by-step explanation:
Hello there!
a. In this case, when zinc metal reacts with bromine, the following chemical reaction takes place:
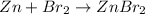
Thus, since zinc and bromine react in a 1:1 mole ratio, we can compute their reacting moles to identify the limiting reactant:
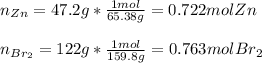
Thus, since zinc has the fewest moles we infer it is the limiting reactant.
b. Here, we compute the grams of zinc bromide via both reactants:
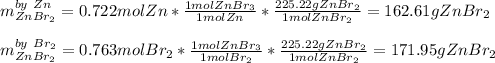
That is why zinc is the limiting reactant, as it yields the fewest moles of zinc bromide product.
c. Here, since just 0.722 mol of bromine would react, we compute the corresponding mass:
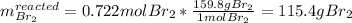
Thus, the leftover of bromine is:
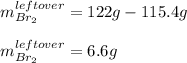
Best regards!