Answer:
31.96°C
Step-by-step explanation:
Given that,
Mass of water, m = 100 g
Initial temperature,
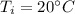
The specific heat capacity of water is 4.18 J/(g℃)
We need to find the final temperature of the water if 5000. J of energy is added to the water in the insulated cup.
The heat added to the water is given by :
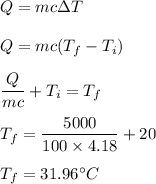
So, the final temperature is 31.96°C.