Answer:
5.04%
Step-by-step explanation:
The accepted value of density = 3.427 g/ml
The calculated value of density = 3.600 g/ml
We need to find Ally's percentage error on her density. The error in any value is given by :
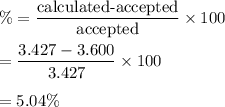
So, the required error is 5.04%.