Answer:
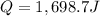
Step-by-step explanation:
Hello!
In this case, since the calculation of the required energy is performed via the following equation:
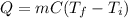
Thus, since the mass is 14 g, specific heat is 4.184 J(g*°C) and the temperatures are 47 °C and 18 °C respectively, the resulting energy is:
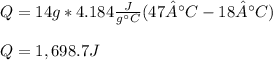
Best regards!