Answer:
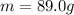
Step-by-step explanation:
Hello!
In this case, since we are given the formula units (molecules) of copper sulfate, it is possible to compute the moles of this compound via the Avogadro's number:
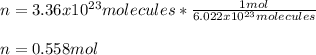
Now, given the molar mass of copper sulfate which is 159.60 g/mol, the required mass in grams turns out:
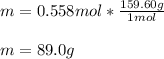
Best regards!