Answer:
15 g
Step-by-step explanation:
The reaction that takes place is:
First we convert 25 g of KCIO₃ to moles, using its molar mass:
- 25 g ÷ 122.55 g/mol = 0.204 mol KCIO₃
Then we convert KCIO₃ moles to KCl moles, using the stoichiometric coefficients:
- 0.204 mol KCIO₃ *
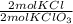 = 0.204 mol KCl
= 0.204 mol KCl
Finally we convert KCl moles to grams, using the molar mass of KCl:
- 0.204 mol KCl * 74.55 g/mol = 15.2 g