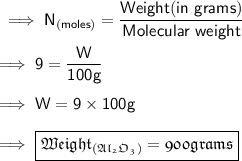
Answer:

Explanation:
Here we are given 9 moles of
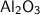 and we need to find its weight in grams . So we know the formula to find the number of moles as ,
and we need to find its weight in grams . So we know the formula to find the number of moles as ,
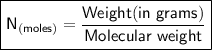
Now the molecular mass of
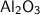 is , equal to 26*2 + 16*3 = 52 + 48 = 100 g
is , equal to 26*2 + 16*3 = 52 + 48 = 100 g
Using the formula to find the Number of moles :-