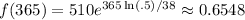Answer:
About 0.6548 grams will be remaining.
Explanation:
We can write an exponential function to model the situation. The standard exponential function is:
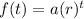
The original sample contained 510 grams. So, a = 510.
Each half-life, the amount decreases by half. So, r = 1/2.
For t, since one half-life occurs every 38 days, we can substitute t/38 for t, where t is the time in days.
Therefore, our function is:
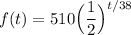
One year has 365 days.
Therefore, the amount remaining after one year will be:
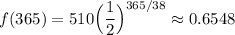
About 0.6548 grams will be remaining.
Alternatively, we can use the standard exponential growth/decay function modeled by:
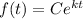
The starting sample is 510. So, C = 510.
After one half-life (38 days), the remaining amount will be 255. Therefore:
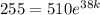
Solving for k:
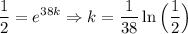
Thus, our function is:
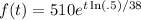
Then after one year or 365 days, the amount remaining will be about: