Answer:
n = 6.06 x
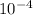 mol
mol
Step-by-step explanation:
n =?
m = 3.06 x 10-³ g
M (H5) = 5 x 1.01 (Since we only want hydrogen)
Atomic mass of C = 12.01
Atomic mass of H is 1,01, etc.
Having this data, we can use the Molar mass formula and change it so we can know the quantity of matter (n) in moles, and we just replace it.
M =
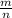 ⇔ n =
⇔ n =
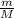 ⇔ n =
⇔ n =
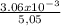 ⇔ n = 6.06 x
⇔ n = 6.06 x
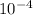 mol
mol
Note: The numbers I've used may be different from yours, by a small difference. I don't know if it's the case, but hope it helped.