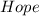Answer:
The rate equation for the general reaction 2A + 2B + 3C → 4D + 5E can be written as:
![Rate = k[A]^x[B]^y[C]^z](https://img.qammunity.org/2024/formulas/mathematics/high-school/svhu1iho2rws2khrhmfi0m22gcofbr1xm5.png)
where:
- [A], [B], and [C] are the concentrations of the reactants A, B, and C, respectively
- [A]^x represents the order of the reaction with respect to A
- [B]^y represents the order of the reaction with respect to B
- [C]^z represents the order of the reaction with respect to C
The values of x, y, and z can be determined experimentally.