Answer:
Hello the table required is missing attached below is the missing table
Answer : 1299.05 k
Step-by-step explanation:
Given data :
concentration of carbon ( Cx ) at a position 3.9mm below surface = 0.35
concentration of carbon ( Co ) = 0.20
surface carbon ( Cs ) = 1
Determine the temperature at which the treatment was carried out
first we will determine the value of Z in the table attached
given that the value of erf ( Z ) for Z = 0.8125 from the table
=
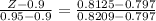
make Z subject of the equation
Z = 0.932
next calculate the diffusion coefficient using the relation below
Z =
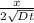 ----- ( 1 ) where ; z = 0.932 , x = 3.9 mm , t = 48h
----- ( 1 ) where ; z = 0.932 , x = 3.9 mm , t = 48h
0.932 =
x = 3.9 mm = 0.0039 m
t = 48 h = ( 48 *60* 60 ) = 172800 secs
Insert values into equation 2
0.932 =
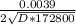
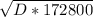 = (0.0039 / 0.932 ) / 2
= (0.0039 / 0.932 ) / 2
172800 * D =( 0.0021 )^2
therefore D = 2.55 * 10^-11 m^2/s
Finally calculate the temperature at which the treatment was carried out
D =
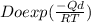 ----- ( 3 )
----- ( 3 )
D = 2.55 * 10^-11 m^2/s ,
Do = 2.3*10^-5 m^2/s ( gotten from Diffusion data table )
Qd = 148000 J/mol ( gotten from Diffusion data table )
R = 8.31 J/mol k ( gotten from Diffusion data table )
back to equation 3
D / Do = exp ( -17810 / T )
1.11 * 10^-6 = exp ( -17810 / T )
therefore T = -17810 / ln( 1.11 * 10^-6 )
= - 17810 / -13.71 = 1299.05 k