Answer: 0.657 L or 657 mL
Step-by-step explanation:
The dilution equation is M₁V₁=M₂V₂ where M is the molarity before and after dilution and V is the volume of solvent.
M₁ is 0.130 as that is the molarity before dilution. V₁ is 0.505 L (converting mL to L is a good practice, though not necessary for this particular problem).
M₂, the molarity we want to achieve, is 0.1.
So, plugging into the above equation, we have
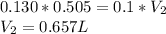
The required volume is 0.657 L, or 657 mL