Answer: 90.04°C
Step-by-step explanation: Calorimeter is a device measures the amount of heat of a chemical or physical process. An ideal calorimeter is one that is well-insulated, i.e., prevent the transfer of heat between the calorimeter and its surroundings. So, the net heat change inside the calorimeter is zero:
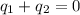
Rearraging, it can be written as
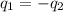
showing that the heat gained by Substance 1 is equal to the energy lost by Substance 2.
In our case, water is gaining heat, because its temperature has risen and so, brass is losing energy:
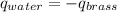
Calculating:
![m_(w).c_(w).\Delta T=-[m_(b).c_(b).\Delta T]](https://img.qammunity.org/2022/formulas/chemistry/college/hv7q9cayjt292n2l7ndbvdvodlnmws18oa.png)
![100.4.18.(18.4-15)=-[52.9.0.375.(18.4-T)]](https://img.qammunity.org/2022/formulas/chemistry/college/8g3b369zfrxyj7kfp95qoarmpzdpzadbs6.png)
Note: final temperature is the same as the substances are in thermal equilibrium.
Solving:
418(3.4)= - 365.01 + 19.8375T
19.8375T = 1786.21
T = 90.04
The initial temperature for the sample of brass was 90.04°.