Answer: a) Temperature of
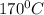
b)
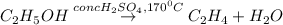
c) dehydrating agent
Step-by-step explanation:
Ethanol is heated with an excess of concentrated sulfuric acid at a temperature of 170°C to produce ethene.
The balanced chemical reaction is:
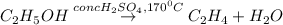
Here concentrated sulfuric acid removes a water molecule from ethanol and thus acts as a dehydrating agent.