Answer:
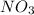
Step-by-step explanation:
Hello!
In this case, since percent compositions are used to set up empirical formulas when assuming those percentages are masses, we can fist compute the moles of nitrogen and oxygen in the compound as shown below:
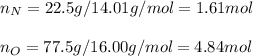
Now, we divide by the fewest moles to compute the subscripts:
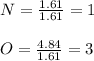
Thus, the empirical formula turns out:
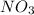
Best regards!