Answer:
[He]2
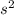 3
3
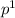
Step-by-step explanation:
The electron configuration for Boron is 1
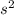 2
2
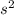 3
3
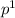 , but you can also write it as the noble gas configuration, which is [He]2
, but you can also write it as the noble gas configuration, which is [He]2
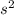 3
3
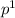
Note that helium has the configuration of 1
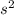 , so we instead of writing it, we write [He]. This can be useful when writing the configurations of elements from the later periods as it saves space.
, so we instead of writing it, we write [He]. This can be useful when writing the configurations of elements from the later periods as it saves space.