Answer:
the flux of sucrose is 9.072 × 10⁻⁷ kg sucrose / m² sec
Step-by-step explanation:
Given the data in the question;
water-gelatin solution separating two different concentration solutions of sucrose; 5.1 wt % gelatin at 293 K; at this conditions diffusivity of sucrose in water gelatin solution is;
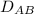 = 0.252 × 10⁻⁹ m²/sec.
= 0.252 × 10⁻⁹ m²/sec.
we know that; 1 L = 0.001 m³, 1mL = 0.001 L
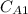 = 2.0 g sucrose/100 mL = 2.0 × 10⁻³ kg sucrose / 100 × 10 ⁻³ L
= 2.0 g sucrose/100 mL = 2.0 × 10⁻³ kg sucrose / 100 × 10 ⁻³ L
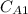 = 2.0kg sucrose / 100 L
= 2.0kg sucrose / 100 L
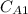 = 2.0 kg sucrose / 100 × 10⁻³ m³
= 2.0 kg sucrose / 100 × 10⁻³ m³
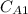 = 20 kg sucrose / m³
= 20 kg sucrose / m³
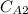 = 0.2 g sucrose/100 mL = 0.2 × 10⁻³ kg sucrose / 100 × 10⁻³ × 10⁻³ L
= 0.2 g sucrose/100 mL = 0.2 × 10⁻³ kg sucrose / 100 × 10⁻³ × 10⁻³ L
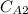 = 2 kg sucrose / m³
= 2 kg sucrose / m³
Thickness ß = 5 mm = 5 × 10⁻³ m
Now, flux of sucrose in kg sucrose / m³sec will be;
using the formula,
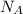 =
=
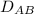 /ß (
/ß (
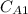 -
-
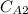 )
)
we substitute
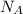 = (0.252 × 10⁻⁹ m²/sec / 5 × 10⁻³ m) ( 20 kg sucrose / m³ - 2 kg sucrose / m³ )
= (0.252 × 10⁻⁹ m²/sec / 5 × 10⁻³ m) ( 20 kg sucrose / m³ - 2 kg sucrose / m³ )
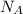 = (0.252 × 10⁻⁶ / 5) × 18 kg sucrose / m² sec
= (0.252 × 10⁻⁶ / 5) × 18 kg sucrose / m² sec
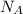 = 4.536 × 10⁻⁶ / 5 kg sucrose / m² sec
= 4.536 × 10⁻⁶ / 5 kg sucrose / m² sec
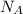 = 9.072 × 10⁻⁷ kg sucrose / m² sec
= 9.072 × 10⁻⁷ kg sucrose / m² sec
Therefore, the flux of sucrose is 9.072 × 10⁻⁷ kg sucrose / m² sec