Answer:
3 Cu + 8 HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
Step-by-step explanation:
Make sure both sides are equal
3 Cu + 8 HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
// start by those elements that change their oxidation degree
Cu and N
// also you can write reduction-oxidation reactions
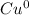 + 2
+ 2
 -->
-->
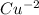 | 2
| 2
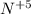 - 3
- 3
 -->
-->
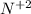 | 3
| 3
// write the numbers of electrons that are lost/gained as the coefficients of the opposite elements
// then check if H and O are the same on both sides
// adjust if they aren't.