Answer:
She will use one 2 as a subscript.
Step-by-step explanation:
Nitrogen is a chemical element with the symbol "N" and has an atomic number of 7. Thus, it is found in group (5) of the periodic table and as such it has 5 electrons in its outermost shell. Therefore, nitrogen has two (5) valence electrons.
On the other hand, oxygen has an atomic number of 8 and with the symbol "O."
When nitrogen and oxygen react chemically, they produce a compound known as nitrogen dioxide
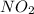
In this scenario, Jenny is studying a compound that has two oxygen atoms and one nitrogen atom. Therefore, the statement which describes the subscripts she will use to write the chemical formula is, she will use one 2 as a subscript.

Where: 2 represents the subscript of oxygen.