To calculate the number of moles of
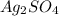 that precipitated, divide the mass of the precipitate by its molar mass. In this example, using 2.00 g of
that precipitated, divide the mass of the precipitate by its molar mass. In this example, using 2.00 g of
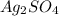 and a molar mass of 311.8 g/mol, the result is approximately 0.00642 moles of
and a molar mass of 311.8 g/mol, the result is approximately 0.00642 moles of
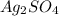 .
.
The process begins with the given mass of precipitate, which is 2.00 grams of
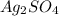 . To determine the number of moles, we use the formula n = m / M, where n is the number of moles, m is the mass of the substance, and M is the molar mass of the substance.
. To determine the number of moles, we use the formula n = m / M, where n is the number of moles, m is the mass of the substance, and M is the molar mass of the substance.
The molar mass of
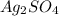 can be calculated by summing the atomic masses of its constituent atoms: 2 × Ag (silver) + 1 × S (sulfur) + 4 × O (oxygen). Using atomic masses from the periodic table, we find M = 2 × 107.87 g/mol + 32.06 g/mol + 4 × 16.00 g/mol ≈ 311.8 g/mol.
can be calculated by summing the atomic masses of its constituent atoms: 2 × Ag (silver) + 1 × S (sulfur) + 4 × O (oxygen). Using atomic masses from the periodic table, we find M = 2 × 107.87 g/mol + 32.06 g/mol + 4 × 16.00 g/mol ≈ 311.8 g/mol.
Substituting the values into the formula, we get n = 2.00 g / 311.8 g/mol ≈ 0.00642 mol. Therefore, approximately 0.00642 moles of
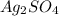 precipitated during the reaction.
precipitated during the reaction.
This calculation is fundamental in stoichiometry, allowing chemists to relate the mass of reactants and products to the number of moles involved in a chemical reaction. It provides a quantitative understanding of the reaction and facilitates accurate analysis and synthesis in chemical processes.