Step-by-step explanation:
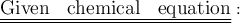
Here , Multiply P2O5 by 2 and O2 by 5 to equalize oxygen :
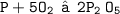
Now , To equalize P atoms , Multiply P by 4
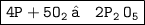
Here , On the left side we have 4P and On the right side ,we have 4P. Again , On the left side , we have 10 O and On the right side , we have 10 O. And yippie , we got the balanced chemical equation !!!
Hope I helped ! ♡
Have a wonderful day / night ! ツ
▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁